|
|
|
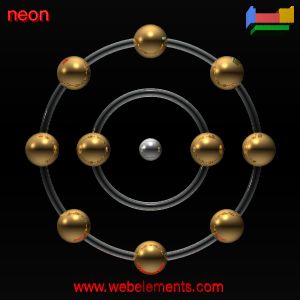
Neon is
a noble gas. Its atomic symbol is Ne. Neon was derived from the Greek word
meaning new, neos. Neons boiling point is -246° C and its cooling point
is
-196° C. Neon was the first
element shown to consist of more than one stable isotope. Neon has many
uses such as in neon signs in which you put neon in and run an electric
charge through it and it causes it to shine as a color. Other uses include
glow lamps,high-voltage indicators, lightning arrestors, wave meter tubes,
TV tubes , electron tubes, plasma studies, fluorescent starter tubes, cryogenic
refrigeration and gas lasers.No chemical compounds that have been observed
that are stable.Neon, a very inert element, is however said to form a compound
with fluorine. It is still questionable if true compounds of neon exist,
but evidence is coming up to support that fact . The ions, Ne+, NeAr+,
NeH+, and HeNe+ are known from optical and mass spectrometric studies.
It also forms an unstable hydrate.
History
Neon was discoverd by Ramsey and
Travers. Neon is rare in the atmosphere 1 part in 65,000 of air. It is
found by liquefaction of air and also separated from the other gases by
fractional distillation.

| Name | Neon |
| Symbol | Ne |
| # of elementary particle | 10 protons 10 electrons 10 neutrons |
| atomic number | 10 |
| atomic mass | 20.180 |
| number of valence electrons | None - noble gas |
| Boiling Point (°C): | -246 |
| Melting Point (°C): | -248.6 |
| Thermal Conductivity | 0.0001 |
| heatVapor | 0.422 |
| heatFusion | 0.08 |
Name in Other Languages
Latin: Neon
Czech: Neon
Croatian: Neon
French: Neon
German: Neon - r
Italian: Neo
Norwegian: Neon
Portuguese: Neônio
Russian:
Spanish: Neón
Swedish: Neon